Lyophilization, a complex multi-step process
Lyophilization, commonly known as freeze-drying, is a crucial strategy in the pharmaceutical industry for stabilizing drugs, vaccines, antibodies, and other biological materials, extending their shelf life by preventing degradation caused by moisture. The methodology consists of removing water by sublimating ice crystals from frozen material.
In addition, lyophilization may also facilitate sustainability. Freeze-dried samples do not require liquid nitrogen or dry ice for storing and even can be stable at room temperature. This fact reduces significantly the shipping and storage costs.
Since lyophilization is a complex multi-step process, optimizing the cycle is essential to ensure consistent product quality and therapeutic efficacy. While small-molecule drug formulations are often simple, consisting of the active ingredient, water, and sometimes a buffer, manufacturing challenges still arise. Issues like prolonged reconstitution time, high moisture content, or instability are often linked to suboptimal freeze-drying cycles.

The process involves mainly pressure and temperature as critical parameters. This white paper pretends to offer an insight into the freeze-drying process in industry, focusing on the importance of conducting a controlled lyophilization cycle when the scaling up is pretended.
Understanding Lyophilization
Freeze-drying is a commonly used method within the pharmaceutical industry, which is divided as a three-step process. The primary goal is to remove water from a substance after it is frozen and placed under a vacuum, allowing the ice to change directly from solid to vapor without passing through the liquid phase (sublimation) (Figure 1).
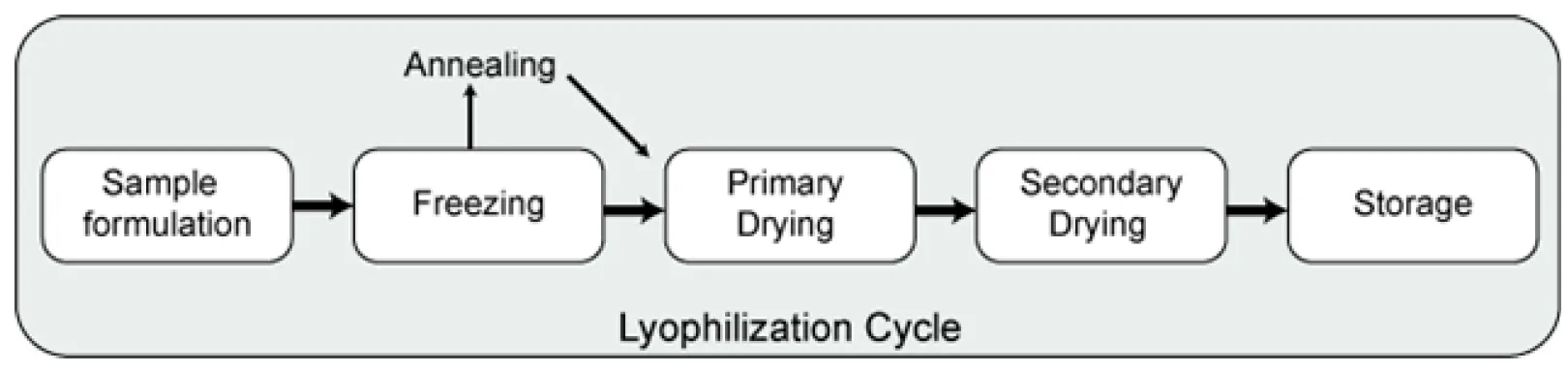
Figure 1. The theoretical framework of lyophilization includes three key steps: an initial freezing phase to transform the liquid solution into a solid state, a primary drying phase where sublimation begins, and a secondary drying phase wherein water desorption takes place.
The shelf temperature and the pressure will control every step during the whole cycle and even in the shelf storing. The temperature changes in the product are monitored by an appropriate temperature sensor placed in a mockup vial containing product (Picture 1).

Picture 1. Example of vial with a wireless sensor
Lyophilization cycle
Typically, the lyophilization cycle consists of three main stages: freezing, primary drying, secondary drying, and, in addition, we can consider storage (Figure 1).
Initially, the product is frozen by lowering the tem- perature below its eutectic or glass transition point to ensure solidification. Controlled cooling rates influence ice crystal formation, impacting drying efficiency. The cycle then progresses to primary drying, where the chamber pressure is reduced through vacuum application, and heat is gradually introduced to sublimate the ice. This step requires careful ramping of temperature to prevent product collapse while maintaining a balance between heat input and mass transfer. Once sublimation is complete, secondary drying begins, further increasing the temperature to remove bound water by desorption, typically at a higher temperature and lower pressure than in primary drying. The final product is then stored under controlled conditions to preserve stability (Figure 2).
Throughout the process, precise control of temperature ramps and pressure transitions is crucial for achieving a reproducible and efficient lyophilization cycle.
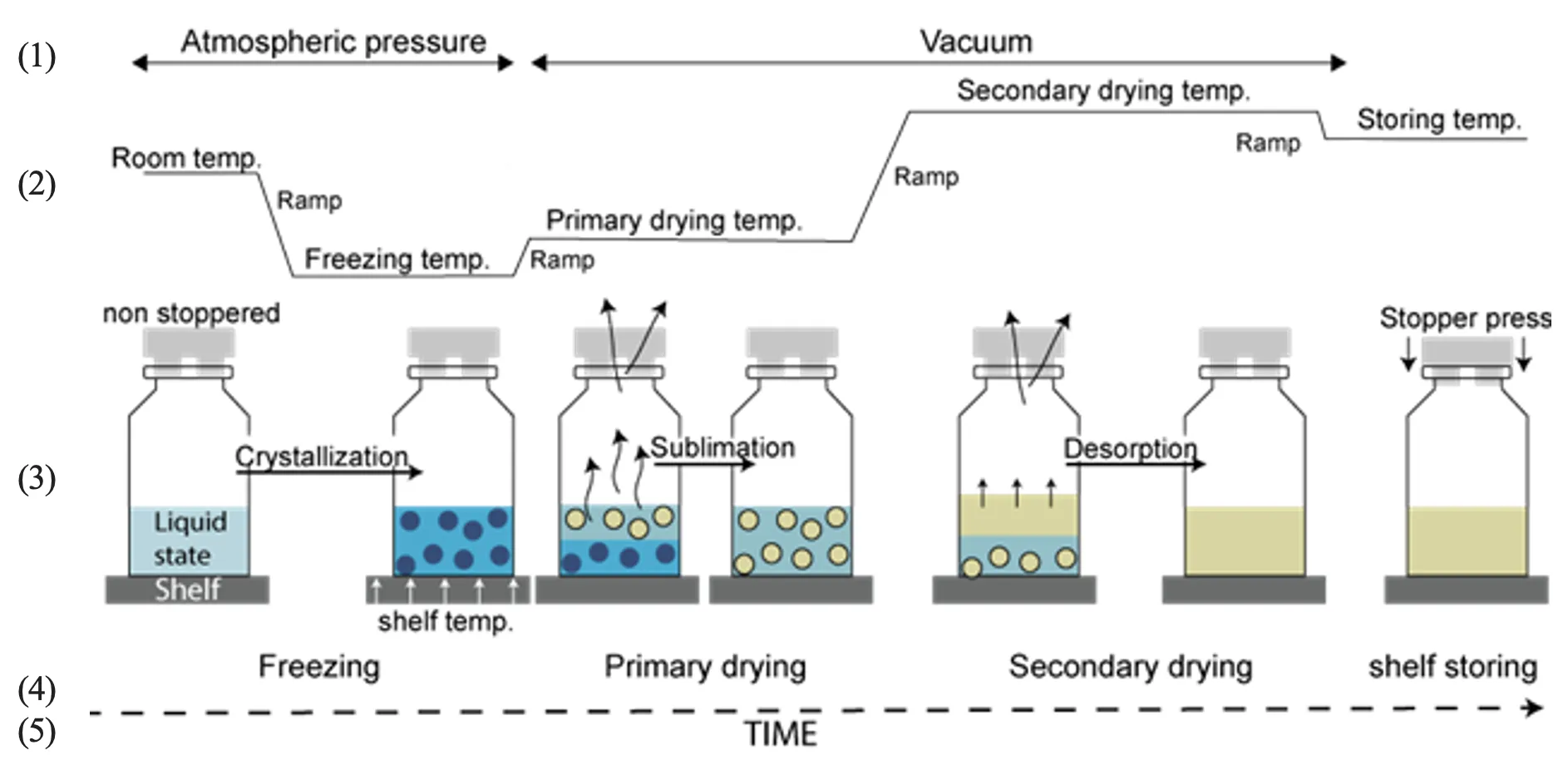
Figure 2. Schematic illustration of lyophilization process in which pressure into the chamber (1), temperature cycle (2), effect into the product (3), lyophilization step (4) and time (5) are represented.
The principle of freeze/sublimation-drying
Freeze-drying is based on the physical principle of the water triple point, where water can coexist in solid, liquid, and gaseous states at atmospheric pressure (~1,000 mbar). However, below the triple point (or eutectic point, for pure water: 6.1 mbar at 0°C), only the solid and gaseous phases are present, allowing ice to sublimate directly into vapor without passing through the liquid state (Figure 3).
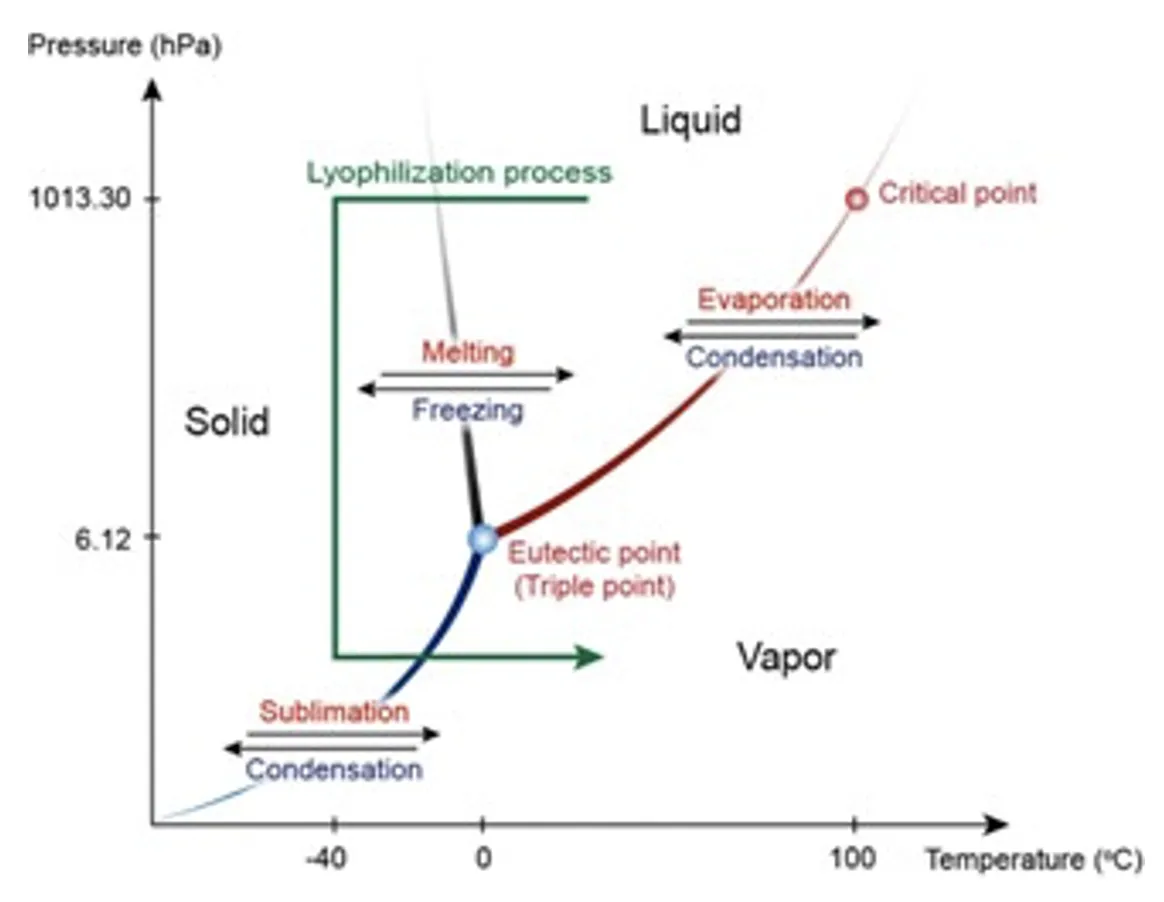
Figure 3. Phase diagram of water. In addition, the ly- ophilization process is indicated (green line). Product is frozen at atmospheric conditions (y axis), then at a temperature below the triple point (x axis), pressure is lowered to reach vapor pressure where ice sublimates.
The ice within the product undergoes direct sublimation into water vapor, bypassing the liquid phase, when the surrounding partial water vapor pressure is lower than the partial pressure of the ice at its respective temperature (Table 1).
The material to be dried is first frozen and then subjected under a high vacuum to heat so that frozen liquid sublimes leaving only solid, dried components of the original liquid. The concentration gradient of water vapor between the drying front and condenser is the driving force for removal of water during lyophilization.

Table 1. Vapor pressure of ice. Note that at very low temperatures lyophilization is impractical.