GMP-Compliant Phage Production: A Scalable Model by Pharmabios
Formulation excipients
Lyophilized formulations usually contain stabilizing excipients, along with bulking agents (Table 2). For instance, the stability of proteins is particularly reliant on their interaction with stabilizers such as sugars through hydrogen bonding. Conversely, the crystallization of stabilizer components or crystalline bulking agents, such as mannitol, during the drying or storage process can adversely affect the stability of the active ingredient [3].
Table 2 presents a comprehensive summary of the most utilized excipients in biologic products.

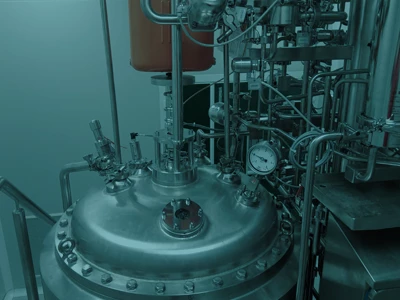
Scaling Up Phage Production Under GMP Standards
Unlike academic-scale phage production, which is often exploratory and limited in scope, GMP-compliant phage therapy production requires robust infrastructure, validated procedures, and full traceability across all stages of the manufacturing cycle. This includes upstream processes like host bacteria cultivation and controlled phage infection, as well as downstream steps such as cell lysis, clarification, purification, and endotoxin removal. Each phase must adhere to Good Manufacturing Practices (GMP) to ensure product consistency, safety, and compliance with regulatory standards. The ultimate goal is to deliver high-quality, clinically viable phage preparations that meet the stringent requirements of therapeutic application—providing a scalable, reliable path from laboratory research to patient-ready solutions.
Core Stages in GMP Phage Manufacturing
- Bacterial Host Cultivation – Grown under sterile, monitored conditions to ensure batch consistency.
- Phage Infection & Lysis – Controlled infection phase where phages are introduced to target bacteria.
- Clarification & Filtration – Removal of cellular debris, producing a clean lysate.
- Endotoxin Removal – Essential to meet therapeutic-grade safety standards.
- Concentration & Formulation – Phages are purified, concentrated, and prepared for final delivery.
- Documentation & Validation – All steps recorded and validated per GMP requirements.

Image 1. Schematic process for GMP phage production in pilot plant
Pharmabios’ Modular Pilot Plant
Pharmabios’ GMP phage therapy production facility is designed to support manufacturing at different scales – from small clinical batches to early commercial stages. The modular architecture ensures flexibility across diverse phage types, allowing for customized production strategies and collaborative validation.
A Collaborative Platform for Innovation
Our model is not a static facility, but a platform built for partnerships. By combining engineering know-how with biotech expertise, Pharmabios offers a space to accelerate development, regulatory compliance, and access to phage-based therapies across Europe and beyond.
Interested in the Full Technical Report?
This blog post is just a summary. If you’d like access to the full extended article with deeper technical insights on GMP phage therapy production, regulatory strategy, and pilot plant setup — just leave us a comment or send a message with the word «PHAGE A1» and we’ll send it to you.